As a person who studies little obscure electric fish, I sometimes wonder why my fish get so little attention in comparison to the more charismatic and popular study organisms. But I shouldn't complain because that's probably nothing in comparison to what plant-studying folks deal with. And that must be nowhere even near what mycologists feel. An entire kingdom severely underrepresented in the scientific community. You'll find the odd mycologist here and there, and luckily for STRI they found Greg Gilbert.

A bit of mushroom science by Greg Gilbert. Nikon D700 + 50mm f/1.4, 1/160, f/2.8, ISO 1600.
We had the pleasure of having Greg as a lecturer during our stay at BCI. He started off by giving us a primer on fungi, where we learned about things such as heart rot in trees (and butt rot of course). After an initial infection through the roots of the tree, the fungi reach the heart of the tree and start a slow digestion process that takes years. Eventually, with its core eaten away, the tree, hollow and structurally weak, snaps and dies. To answer the remaining mysteries of this process, such as which trees are more susceptible to these infections or how prevalent fungal pathogens are, or even how specific are fungal pathogens to their hosts, we need someone like Greg to come in and show us how to see through trees.
Step 1. Select tree of choice.
Step 2. Look inside tree. Tadaaa!
Maybe step 2 is a bit oversimplified. It involves using a hammer, an even fancier digital hammer, twelve flattened thumbtacks and the coolest biggest digital calliper. Unfortunately I don't have any images of it so just imagine giant tweezers with buttons, bluetooth, and independently moving arms. After selecting the tree and measuring its perimeter, we hammered down 12 evenly spaced thumbtacks around the tree. Then, using the calliper, we measured distances between thumbtack pairs. Meanwhile, on an automated computer software, each of these distances was used to construct a digital cross-section of the target tree. Now that we had the overall shape, we used sonic and electric impedance tomography to see inside the tree.
Hammer. Nikon D700 + 50mm f/1.4, 1/60, f/2.5, ISO 1600.

12 thumbtacks placed around the tree. Nikon D700 + 50mm f/1.4, 1/60, f/2.5, ISO 1600.
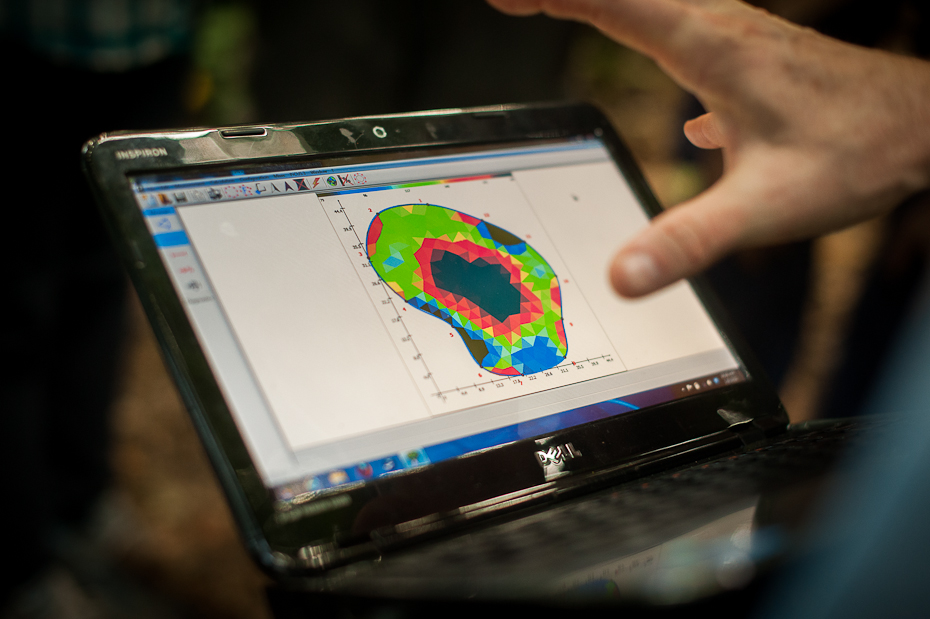
Let's start with sonic tomography. If a tree is hollow, sonic vibrations require more time to pass through the tree and reach the other side. Using a hammer, this time the fancier digital one, we smacked smacked thumbtacks one by one while the software recorded the exact time it took for the sound of impact to reach each of the other thumbtacks. Knowing the distance between thumbtacks and the time it took for sound to travel, the computer software determines whether the space between the tacks is dense or hollow. This information is compiled and a few moments later, an image of the inside of the trunk is generated. In our tree, evidence of a fungal pathogen infection was manifested as a large hole in the tree's cross-section.
The fancier hammer. Nikon D700 + 50mm f/1.4, 1/60, f/2.5, ISO 1600.
Owen smacking one of the 12 tacks. Nikon D700 + 20mm f/2.8, 1/80, f/2.8, ISO 1600.
Electric impedance tomography. Nikon D700 + 50mm f/1.4, 1/160, f/2.2, ISO 1600.
Results of our electric impedance tomography. Nikon D700 + 50mm f/1.4, 1/160, f/2.2, ISO 1600.
With electric impedance tomography, we are seeing through the tree not by using sound but rather current. Although it may seem like we're trying to blow the tree up, we're calculating the resistance between each tack pairs, which will give us an idea of the water content distribution in the tree. This technique allows us to distinguish cavities from wet diseased wood. The two cutting-edge techniques, often used in conjunction, will allow scientists like Greg Gilbert to understand how fungal pathogens are affecting different forest tree diversity.